Team
| Name | Contact | |
|---|---|---|

| Dr. Daniela Goedderz | daniela.goedderz@lbf.fraunhofer.de +49 6151 705-8914 |

| Thomas Driever M.Sc. | thomas.driever@lbf.fraunhofer.de |
Motivation
We investigate the mechanisms of action of selected flame retardants in an unsaturated polyester resin matrix, which have so far been insufficiently researched, especially with regard to the interaction between flame and flame retardant. The scientific knowledge gained will accelerate the development of future formulations for the efficient use of flame retardants and support a scientific evaluation of the effectiveness and mode of action of flame retarded polymers.
Objectives
The aim is to experimentally investigate the mechanisms of action of phosphorus-based flame retardants in detail. In collaboration with other projects of the TRR150, this enables the formulation of a reaction mechanism for the selected flame retardants. The reaction mechanism must be specifically formulated taking into account application-specific aspects such as the fire behavior of the resin system with the selected flame retardant and thus the flame retardant and carrier material should be viewed as an overall system.
The combustion behavior of a flame retarded material is determined, among other things, by the interaction of the polymer and the flame retardant respectively its decomposition products. The effectiveness of a flame retardant is therefore always linked to the polymer matrix used or examined. In contrast to the literature, the planned collaborations will combine methods that enable a more comprehensive look at the mechanisms of action than before. The resulting structure-property relationships will support the targeted and efficient use of flame retardants in future. The transferability of the developed methodology to different flame retardants and polymer matrices is being tested. If successful, these findings will accelerate the formulation development of flame retarded polymer mixtures significantly.
Previous Findings
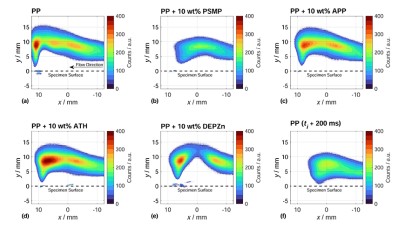
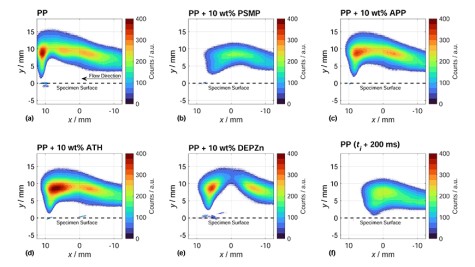
Geschwindner and Goedderz et al. used an interdisciplinary methodical approach to investigate the modes of action of flame retardants in the gas phase during the combustion process of a polymer. High-performance flame retardant formulations usually consist of different mixtures of flame retardants so that different modes of action can be combined. In order to study the chemical and physical processes during combustion, it is necessary to understand the mode of action of each individual component in the flame retardant formulation. Classic chemical analysis methods were used to investigate the decomposition process of the flame retarded polymer and to identify typical decomposition products. A major disadvantage of these classic analysis methods is the decoupling of the combustion process. Visualizing the hydroxy radical concentration during combustion using high-speed planar laser-induced fluorescence (OH-PLIF) provides an additional perspective on the combustion process as the hydroxy radical concentration allows an assessment of the effectiveness of the flame retardant in the gas phase.
Various flame retardants were examined and compared in polypropylene (PP) as a simple polymer matrix. The combination of different analysis methods revealed some particularities: The change in flame topology when interacting with an external flame and also during the combustion process. For DEPZn (10wt% in PP) as phosphinate, the release of flammable gases competing with a flame retardant effect could be observed, resulting in small jet-like flames in the flame zone, while clear gas phase activity could be observed. PSMP as phosphonate showed a very effective ability to reduce the hydroxy radical concentration in the gas phase. ATH and APP were not able to reduce the hydroxy radical concentration in the gas phase as effectively as DEPZn and PSMP as a result of the predominantly condensend phase activity.
Approach
The base polymer matrix used is a bio-based UP resin combined with a reactive diluent (e.g. styrene or bio-based alternatives) that can be used to control the processing viscosity of the system. In addition, both liquid and solid flame retardants can be incorporated.
The combustion behavior of the polymer matrix with and without flame retardant is investigated using several analytical methods. First, common chemical analysis tools such as nuclear magnetic resonance spectroscopy, infrared spectroscopy and SEM are used to investigate the residue from burning experiments. By identifying elements or decomposition products, it is possible to draw conclusions about the chemical and physical processes occurring in the solid phase. To investigate the gas phase and the combustion behavior in general, we use a Cone Calorimeter and thermogravimetric analysis. In addition, to detect active radical species in the gas phase, an FTIR device is coupled to the Cone Calorimeter enabling a completely new study of processes occurring in the gas phase of a fire retarded polymer. Lastly, to understand the decomposition of the selected flame retardants, various of mass spectrometry methods are used to draw a decomposition mechanism of the flame retardants with and without the polymer matrix.
Cooperation
By identifying and analyzing the respective decomposition processes of the flame retardants and flame retarded polymers, C08 provides a basis for B04, B05 and B06, in which the determined data are required for the characterization of the elementary steps in the gas and solid phase with regard to a kinetic and thermodynamic description.
The investigations in the gas phase focus on the chain termination reactions that take place, which are the cause of flame inhibition and in which the hydroxy radical plays a central role as a reactive species. These processes are examined in close coordination with B04. In the solid phase, together with B05 a model is developed to analyze the complex processes that occur between flame retardants and the polymer matrix and their decomposition products. In addition, the investigation of various phosphorus-containing species (e.g. phosphonates, aromatic/aliphatic, metal phosphinates, phosphates), structure-property relationships can be taken into account and central structural units for the flame retardancy of the polymer system can be identified. In a fire scenario together with C06, the effects of flame retardants and flame retarded polymers on a boundary layer flame are investigated. The hydroxy radical concentration and the local thermochemical states are important parameters for evaluating the gas phase activity of flame retardants and their flame inhibition. The influence of the polymer matrix on the mechanism of action can be illustrated by comparing differently designed active walls consisting of different flame retarded polymer formulations.